Example Experiment: Characterizing ERK Activation in Response to Phorbol 12-Myristate 13-Acetate (PMA)
Background
Accurate quantification of protein expression and/or post-translational modifications is important for advancing both basic and translational research. Given the flexibility, reproducibility, and higher throughput of the In‑Cell Western™ Assay, it offers a convenient alternative to Western blotting and is a powerful platform for meaningful in situ analyses. The In-Cell Western microplate format can be used to analyze:
- Protein phosphorylation and signaling (1 - 3)
- Off-target effects of drugs on signaling pathways (4)
- Timing and kinetics of signaling events (5, 6)
- Quantification of viral load (7 - 11)
- Genotoxicity assays (12, 13)
- Cell proliferation and apoptosis assays (14)
- Bacterial-induced epithelial signaling (15)
- Glycoprotein analysis (16, 17)
- Library screening (18 - 20)
- Screening of monoclonal antibody clones (21)
Introduction
The protein kinase C (PKC) pathways regulate numerous processes such as, cellular proliferation, gene expression, and the inflammatory response (22). Due to the multifaceted roles of the PKC signaling pathway, it is not surprising that the perturbation of this pathway can promote malignant behavior in cancer cells, such as increased cell survival, proliferation, migration, and the epithelial-mesenchymal transition (23). Therefore, exploring PKC and its corresponding signaling pathways can provide critical knowledge in understanding its role in both normal and pathological processes.
Experimental Design
In the following In-Cell Western example, we present a protocol for monitoring ERK phosphorylation in response to stimulation by phorbol 12-myristate 13-acetate (PMA), a potent nanomolar activator of protein kinase C (PKC) (24). PMA acts by binding to the C1 domain of PKC, thereby triggering a signal transduction cascade that initiates a variety of biochemical and molecular changes, including the phosphorylation and activation of ERK (25, 26). Changes in phospho- and total-ERK protein levels are detected and characterized in 3 suspension cell lines (Jurkat, K-562, and THP-1) treated with a serial dilution of PMA. The information obtained from this experimental protocol could be used to identify appropriate experimental conditions to screen for and characterize compounds that modulate this branch of the PKC signaling pathway.
Required Reagents
LI‑COR Reagents
-
IRDye® 800CW Goat anti-Rabbit Secondary Antibody (LI-COR P/N 925-32211 or 926-32211)
-
IRDye 680RD Goat anti-Mouse Secondary Antibody (LI-COR P/N 925-68070 or 926-68070)
-
Intercept® (PBS) Blocking Buffer
Odyssey® Blocking Buffer was used in the original experiment and has been discontinued. Intercept Blocking Buffer is now available instead of Odyssey Blocking Buffer.
-
Large Western Incubation Box (LI-COR P/N 929-97301)
Additional Reagents
-
1X PBS wash buffer
-
Tissue culture reagents (RPMI 1640, fetal bovine serum)
-
Black-sided 96-well or 384-well microplates with clear well bottoms
-
Jurkat cells (ATCC®, P/N TIB-152™)
-
THP-1 monocytes (ATCC, P/N TIB-202™)
-
K-562 lymphocytes (ATCC, P/N CCL-243™)
-
Concentrated Prefer (5X) (Anatech LTD, P/N 411)
-
TO-PRO®-3 (Molecular Probes, P/N T-3605)
-
PMA (phorbol 12-myristate 13-acetate) (Sigma®, P/N P1585)
-
DMSO (dimethyl sulfoxide) (Sigma, P/N D8418)
-
ERK rabbit antibody (Santa Cruz, P/N sc-94)
-
pERK mouse antibody (Cell Signaling Technology, P/N 9106)
Prepare Cells
-
Allow Jurkat (ATCC, P/N TIB-152) cell growth in a T75 flask using standard tissue culture procedures. Avoid growing cells to density greater than 2 x 106 cells.
-
Transfer cells in growth media to 50 mL conical tubes and centrifuge at 500 x g for 5 minutes.
-
Remove media and resuspend cell pellet in 10 mL of serum-free media (pre-warmed to 37 °C). Pipet slowly to maintain cell integrity while disrupting the cell pellet. Transfer resuspended cells into T75 flask and place in an incubator (37 °C and 5% CO2).
It is the serum withdrawal from the complete media that allows suspension cells to attach to plates (i.e., T75 flask). Gravity will cause cells to form a monolayer over time (10 to 15 minutes). Once a monolayer is formed, the rest of the cells in the serum-free media will remain in suspension and will not attach to the plates once a monolayer of cells are established. Only the cells in suspension in the T75 flask will be used in the following steps.
-
Allow cells to settle for 30 minutes before taking a 50 µL aliquot of cells for counting using a hemocytometer.
-
Add appropriate volume of serum-free media so that 1 x 106 cells/mL is achieved (1 plate x 96 wells x 200 µL of cells/well = 20 mL/plate).
-
Serum-deprive cells by replacing cells suspended in serum-free media back into the incubator for an additional 3.5 hours or overnight.
Treat Cells
-
Add 2 µL of DMSO for both the background samples (serves as non-specific background fluorescence) and resting cells (serves as basal control) in triplicate wells. Add 2 µL of 1:1 serial dilutions of PMA ranging from 0.005 to 3.2 ng/mL in triplicate wells.
-
Using a multi-channel pipettor, transfer 200 µL of suspended cells (~200,000 cells) per well into the wells containing 2 µL of DMSO or PMA from step 6.
-
Allow incubation at 37 °C for 15 minutes.
Be careful not to disrupt cells during this PMA-induced activation step. During this critical step, cells will sediment to the bottom of the wells by gravity, forming a monolayer. This monolayer can be easily viewed under a light source. The monolayer will appear opaque rather than transparent. Clumping of cells will lead to detachment from plates during incubation and washing steps. Be careful in handling the plate at this stage because the cells will be very loosely attached to the bottom of the wells.
Fix and Permeabilize Cells
Fix Cells
-
Directly add to the cell suspension 50 µL of concentrated (5X) Prefer (or 25 µL of 37% formaldehyde to the cell suspension; final concentration of 4%) into each well.
Gently add Prefer into wells using side of the wells to avoid detaching the cells from the well bottom. During fixation, the cell monolayer will attach more firmly to the wells; however, the strength of the attachment is never as strong as that of adherent cells grown on plates. Therefore, a degree of caution is needed during every step of this procedure.
-
Allow cells to fix for 20 minutes at room temperature with very gentle rotation (set at speed 2 on The Belly Dancer® (Stovall)).
-
Centrifuge at 1,500 rpm (332 rcf) for 10 minutes.
Permeabilize Cells
Wash 3 times with 1X PBS containing 0.1% Triton® X-100 (cell permeabilization) for 5 minutes per wash by centrifugation at 1,500 rpm (332 rcf).
-
Prepare Triton Washing Solution as follows:
1X PBS 495 mL 10% Triton X-100 5 mL 1X PBS + 0.1% Triton X-100 500 mL -
Remove Fixing Solution (if using formaldehyde, collect in an appropriate waste container).
-
Using a multi-channel pipettor, add 100 μL of Triton Washing Solution (room temperature (RT)). Add the solution down the sides of the wells carefully to avoid detaching the cells.
-
Centrifuge at 1,500 rpm (332 rcf) for 5 minutes.
-
Repeat washing steps 2 more times, removing wash manually each time.
Do not allow cells to become dry during washing. Immediately add the next wash after manual disposal.
Block Cells
- Using a multi-channel pipettor, block cells/wells by adding 150 µL of Intercept® Blocking Buffer to each well. Add the solution by pipetting down the sides of the wells carefully to avoid detaching the cells.
- Allow blocking for 1 hour at RT with very gentle shaking on a plate shaker.
Primary Antibodies
Dilute Primary Antibodies
-
Dilute the two primary antibodies in Intercept® Blocking Buffer. Combine the following antibodies for ERK target analysis:
Rabbit anti-ERK antibody (1:200 dilution in the combined solution; Santa Cruz)
Mouse anti-phospho-ERK antibody (1:100 dilution in the combined solution; Cell Signaling Technology)
-
Mix the primary antibody solution thoroughly before adding to wells.
Incubate with Primary Antibodies
- Remove blocking buffer and add 50 µL of the desired primary antibody or antibodies in Intercept Blocking Buffer to cover the bottom of each well.
- Make sure to include control wells without primary antibody to serve as a source for background well intensity. Only add 50 µL of Intercept Blocking Buffer to control wells.
- Incubate with primary antibody for 2 hours with gentle shaking at RT.
Wash
-
Prepare Tween® Washing Solution as follows:
1X PBS 995 mL 20% Tween 20 5 mL 1X PBS + 0.1% Tween 20 1000 mL -
Remove primary antibody solution.
-
Using a multi-channel pipettor, add 200 µL Tween Washing Solution (RT). Add solution down the sides of the wells carefully to avoid detaching the cells from the well bottom.
-
Centrifuge at 1,500 rpm (332 rcf) for 5 minutes.
-
Gently remove Tween Washing Solution by manually pipetting.
-
Repeat washing steps 4 more times.
Secondary Antibodies
Dilute Secondary Antibodies
-
Dilute the fluorescently-labeled secondary antibodies in Intercept® Blocking Buffer as specified below. To lower background, add Tween® 20 to the diluted antibody to a final concentration of 0.2%. Recommended dilution range is 1:200 – 1:1,200.
Goat anti-Mouse IRDye® 680RD (1:800 dilution in the combined solution)
Goat anti-Rabbit IRDye 800CW (1:800 dilution in the combined solution)
Minimize exposure of the antibody vials to light.
-
Mix the antibody solutions and add 50 µL of the secondary antibody solution to each well.
Incubate with Secondary Antibodies
-
Incubate for 60 minutes with gentle shaking at RT. Protect plate from light during incubation.
Use a large Western Incubation Box to protect plate from light during subsequent steps.
Wash
- Remove secondary antibody solution.
- Using a multi-channel pipettor, add 200 µL of Tween Washing Solution at RT (Wash). Add solution down the sides of the wells carefully to avoid detaching the cells from the well bottom.
- Centrifuge at 1,500 rpm (332 rcf) for 5 minutes.
- Gently remove Tween Washing Solution by manually pipetting.
- Repeat washing steps 4 more times. Protect plate from light during washing.
Image
- After final wash, remove wash solution completely from wells. Turn the plate upside down and tap or blot gently on paper towels to remove traces of wash buffer. For best results, scan plate immediately; plates may also be stored at 4 °C for several weeks (sealed and protected from light).
- Before plate scanning, clean the bottom plate surface and the Odyssey® Imager scanning bed (if applicable) with moist, lint-free tissue to avoid any obstructions during scanning.
- Scan plate with detection in both 700 and 800 nm channels.
Suggested Scan Settings
All settings may require adjustment for optimal data quality. Higher resolutions or scan qualities can be used, but the scan time will increase.
| Instrument | Resolution | Scan Quality | Intensity Setting (700 nm) | Intensity Setting (800 nm) |
| Odyssey Classic | 169 µM | lowest | 5 | 5 |
| Odyssey DLx | 169 µM | lowest | Auto Mode | Auto Mode |
Experimental Results
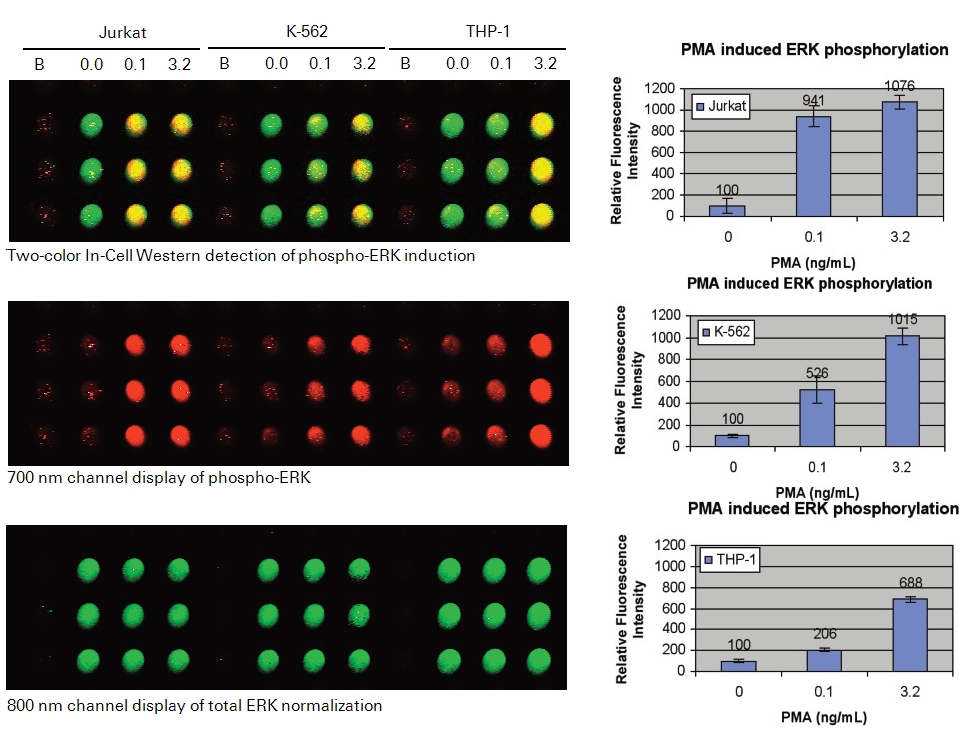
References
1. Chen, H., Kovar, J., Sissons, S., Cox, K., Matter, W., Chadwell, F., Luan, P., Vlahos, C. J., Schutz-Geschwender, A., and Olive, D. M. (2005) A cell-based immunocytochemical assay for monitoring kinase signaling pathways and drug efficacy. Analytical biochemistry 338, 136-142
2. Aguilar, H. N., Zielnik, B., Tracey, C. N., and Mitchell, B. F. (2010) Quantification of rapid Myosin regulatory light chain phosphorylation using high-throughput in-cell Western assays: comparison to Western immunoblots. PLoS One 5, e9965
3. Wong, S. K. (2004) A 384-well cell-based phospho-ERK assay for dopamine D2 and D3 receptors. Analytical biochemistry 333, 265-272
4. Kumar, N., Afeyan, R., Kim, H. D., and Lauffenburger, D. A. (2008) Multipathway model enables prediction of kinase inhibitor cross-talk effects on migration of Her2-overexpressing mammary epithelial cells. Mol Pharmacol 73, 1668-1678
5. Hannoush, R. N. (2008) Kinetics of Wnt-driven beta-catenin stabilization revealed by quantitative and temporal imaging. PLoS One 3, e3498
6. Chen, W. W., Schoeberl, B., Jasper, P. J., Niepel, M., Nielsen, U. B., Lauffenburger, D. A., and Sorger, P. K. (2009) Input-output behavior of ErbB signaling pathways as revealed by a mass action model trained against dynamic data. Mol Syst Biol 5, 239
7. Counihan, N. A., Daniel, L. M., Chojnacki, J., and Anderson, D. A. (2006) Infrared fluorescent immunofocus assay (IR-FIFA) for the quantitation of non-cytopathic and minimally cytopathic viruses. J Virol Methods 133, 62-69
8. Lin, Y. C., Li, J., Irwin, C. R., Jenkins, H., DeLange, L., and Evans, D. H. (2008) Vaccinia virus DNA ligase recruits cellular topoisomerase II to sites of viral replication and assembly. J Virol 82, 5922-5932
9. Weldon, S. K., Mischnick, S. L., Urlacher, T. M., and Ambroz, K. L. (2010) Quantitation of virus using laser-based scanning of near-infrared fluorophores replaces manual plate reading in a virus titration assay. J Virol Methods 168, 57-62
10. Lopez, T., Silva-Ayala, D., Lopez, S., and Arias, C. F. (2012) Methods suitable for high-throughput screening of siRNAs and other chemical compounds with the potential to inhibit rotavirus replication. J Virol Methods 179, 242-249
11. Wan, Y., Zhou, Z., Yang, Y., Wang, J., and Hung, T. (2010) Application of an In-Cell Western assay for measurement of influenza A virus replication. J Virol Methods 169, 359-364
12. Jamin, E. L., Riu, A., Douki, T., Debrauwer, L., Cravedi, J. P., Zalko, D., and Audebert, M. (2013) Combined genotoxic effects of a polycyclic aromatic hydrocarbon (B(a)P) and an heterocyclic amine (PhIP) in relation to colorectal carcinogenesis. PLoS One 8, e58591
13. Khoury, L., Zalko, D., and Audebert, M. (2013) Validation of high-throughput genotoxicity assay screening using gammaH2AX in-cell western assay on HepG2 cells. Environ Mol Mutagen 54, 737-746
14. Godin-Heymann, N., Ulkus, L., Brannigan, B. W., McDermott, U., Lamb, J., Maheswaran, S., Settleman, J., and Haber, D. A. (2008) The T790M "gatekeeper" mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther 7, 874-879
15. Du, Y., Danjo, K., Robinson, P. A., and Crabtree, J. E. (2007) In-Cell Western analysis of Helicobacter pylori-induced phosphorylation of extracellular-signal related kinase via the transactivation of the epidermal growth factor receptor. Microbes Infect 9, 838-846
16. McInerney, M. P., Pan, Y., Short, J. L., and Nicolazzo, J. A. (2017) Development and Validation of an In-Cell Western for Quantifying P-Glycoprotein Expression in Human Brain Microvascular Endothelial (hCMEC/D3) Cells. J Pharm Sci 106, 2614-2624
17. Urlacher T, Xing K, Cheung L et al (2013) Glycoprotein applications using near-infrared detection. Poster presentation, Experimental Biology
18. Guo, K., Shelat, A. A., Guy, R. K., and Kastan, M. B. (2014) Development of a cell-based, high-throughput screening assay for ATM kinase inhibitors. J Biomol Screen 19, 538-546
19. Hoffman, G. R., Moerke, N. J., Hsia, M., Shamu, C. E., and Blenis, J. (2010) A high-throughput, cell-based screening method for siRNA and small molecule inhibitors of mTORC1 signaling using the In Cell Western technique. Assay Drug Dev Technol 8, 186-199
20. Schnaiter, S., Furst, B., Neu, J., Waczek, F., Orfi, L., Keri, G., Huber, L. A., and Wunderlich, W. (2014) Screening for MAPK modulators using an in-cell western assay. Methods Mol Biol 1120, 121-129
21. Daftarian, M. P., Vosoughi, A., and Lemmon, V. (2014) Gene-based vaccination and screening methods to develop monoclonal antibodies. Methods Mol Biol 1121, 337-346
22. Newton, A. C. (2018) Protein kinase C: perfectly balanced. Crit Rev Biochem Mol Biol 53, 208-230
23. Garg, R., Benedetti, L. G., Abera, M. B., Wang, H., Abba, M., and Kazanietz, M. G. (2014) Protein kinase C and cancer: what we know and what we do not. Oncogene 33, 5225-5237
24. Castagna, M., Takai, Y., Kaibuchi, K., Sano, K., Kikkawa, U., and Nishizuka, Y. (1982) Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 257, 7847-7851
25. Ono, Y., Fujii, T., Igarashi, K., Kuno, T., Tanaka, C., Kikkawa, U., and Nishizuka, Y. (1989) Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci U S A 86, 4868-4871
26. Ueda, Y., Hirai, S., Osada, S., Suzuki, A., Mizuno, K., and Ohno, S. (1996) Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem 271, 23512-23519