NewBlot™ PVDF 5X Stripping Buffer
Components
NewBlot PVDF 5X Stripping Buffer (P/N 928-40032): 100 mL
Specifications
- Size: 100 mL (for up to 3,000 cm2 or approximately fifty 7 x 8.5 cm Millipore Immobilon®-FL PVDF membranes)
- Storage: Room temperature
Reagents Needed
- 1X PBS wash buffer
- Ultrapure water
- Large incubation box, such as LI-COR® Incubation Box (11.75 x 8.9 cm, P/N 929-97301)
- Completed Western blot to be stripped (wet)
Guidelines
- NewBlot PVDF 5X Stripping Buffer has been optimized for use with IRDye antibody conjugates and Millipore Immobilon-FL PVDF membrane.
- NewBlot PVDF 5X Stripping Buffer has been optimized for use with IRDye® 800CW, IRDye 680LT, and IRDye 680RD conjugates.
- LICORbio neither guarantees nor supports the use of NewBlot PVDF 5X Stripping Buffer with PVDF membranes or antibody-dye conjugates from other manufacturers.
- NewBlot PVDF 5X Stripping Buffer has not been tested for use with chemiluminescent detection or colorimetric substrates.
Stripping Guidelines
Familiarize yourself with the entire document before proceeding. Ensure that you have the necessary supplies and reagents for stripping the membrane.
General
- Stripping efficacy on Odyssey PVDF membranes and the number of times a membrane can be stripped are primarily affected by time, concentration, and temperature.
- The number of times a membrane can be stripped is also dependent on other factors, such as the type and amount of bound antigen, the type of membrane used, and the stringency of stripping conditions. Typically, a membrane can be stripped up to three times.
Reprobing
- Increasing the stripping time and the stripping buffer concentration will progressively reduce the ability to successfully reprobe the blot due to the increased likelihood of damage to the target antigen. The Optimization Protocol can help determine the appropriate stripping concentration for the blot and improve the removal of fluorescent signal.
- Increasing the temperature can significantly improve stripping effectiveness but can also have a highly detrimental effect on reprobing. If results are inadequate after completing the protocol, stripping buffer incubation can be carried out at 37 °C using a water bath or warm air incubator.
Stripping Buffer Working Solution
- Always prepare fresh stripping buffer working solution for each blot that will be stripped.
-
Discard the stripping buffer working solution once further stripping of the membrane is no longer needed.
Protocol
Keep the blot moist at all times. If it dries during the incubation, washing, scanning, or stripping step, it can reduce the ability to successfully strip and reprobe the blot.
-
Prepare a 1X working solution of NewBlot™ PVDF 5X Stripping Buffer by mixing one part stripping buffer with four parts water. Optimization of the working solution concentration may be required.
A large LI-COR® incubation box with a 7 x 8.5 cm membrane requires a minimum of 20 mL of working solution.
-
Transfer the working solution to a clean incubation container.
-
Place the blot into the incubation container. Ensure it is fully submerged and can move freely within the container. Place the container on a rotator and shake briskly (70-80 rpm) for 20 minutes at room temperature.
-
Immediately rinse the blot by removing it from the stripping buffer and completely submerging it in a fresh container of 1X PBS. Repeat rinse two more times, using fresh 1X PBS each time.
Allow the blot to remain in 1X PBS after the last rinse until further processing. Do not allow the blot to dry.
-
Image the blot on an Odyssey family imager to ensure the complete removal of sample fluorescence.
-
If sample fluorescence signal remains, refer to the Optimization Protocol. If not, proceed to reprobe the blot with the desired primary and secondary antibodies.
Optimization Protocol
To determine the starting concentration of stripping buffer that is needed for the experimental blot, first perform the following steps on a test blot that will not be analyzed.
- Place the blot back into the 1X working solution of NewBlot PVDF 5X Stripping Buffer. Place container onto a rotator and shake briskly for 10 minutes at room temperature.
- Immediately rinse the blot by removing it from the stripping buffer and completely submerging it in a fresh container of 1X PBS. Repeat rinse two more times, using fresh 1X PBS each time.
- Image the blot on an Odyssey family imager to ensure antibodies and fluorescent signal have been completely removed.
If fluorescent signal remains after imaging:
- Add Sodium Dodecyl Sulfate (SDS) to the stripping buffer container to a final concentration of 0.5% (v/v) then mix. Place the blot into the 1X Buffer + SDS solution then shake briskly for 5 minutes (room temperature).
- Immediately rinse by removing the blot from the stripping buffer and placing it into a fresh container of 1X PBS. Repeat rinse two more times; use fresh 1X PBS each time.
- Reimage the blot on an Odyssey family imager to ensure antibodies and fluorescent signal have been completely removed.
- If fluorescent signal remains, incubation time can be extended in increments of 5 or 10 minutes until desired results are achieved.
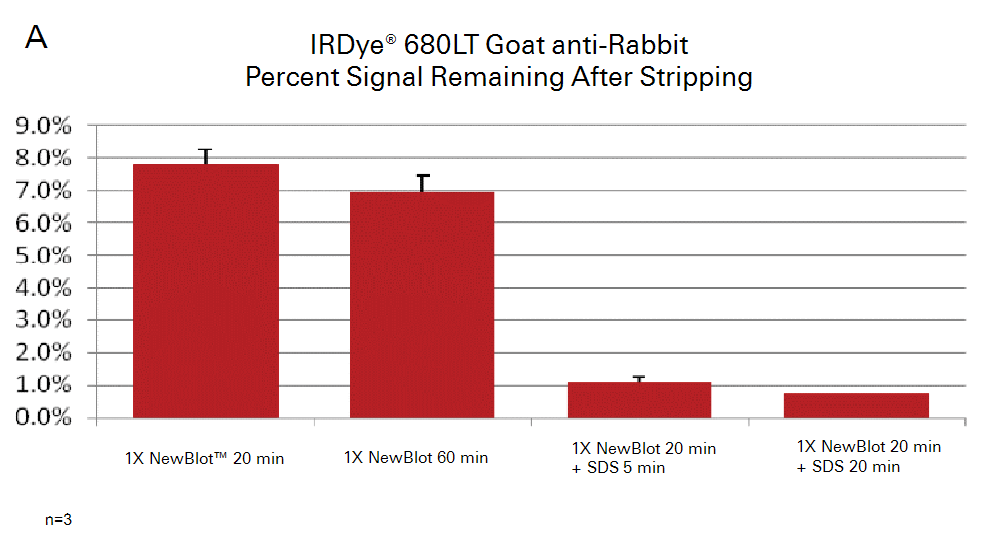
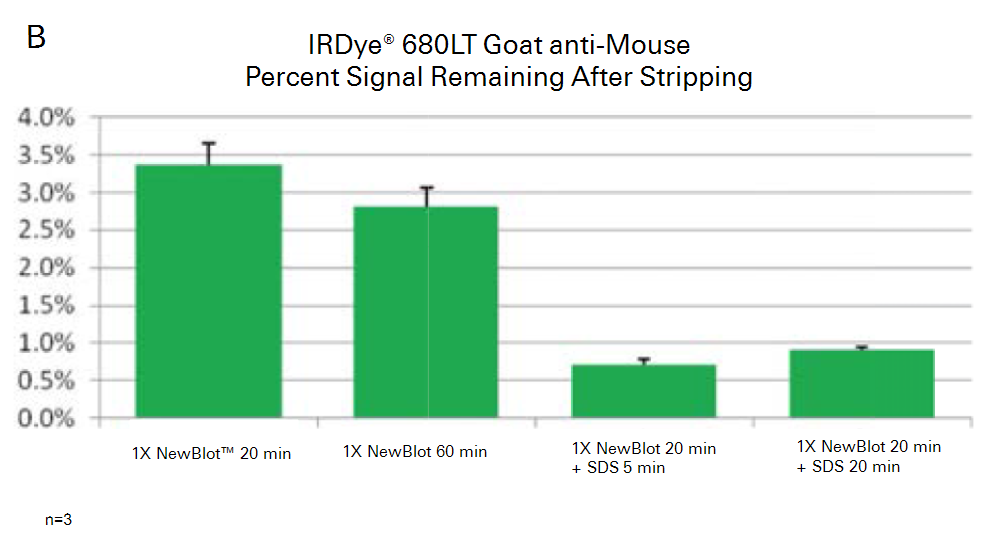
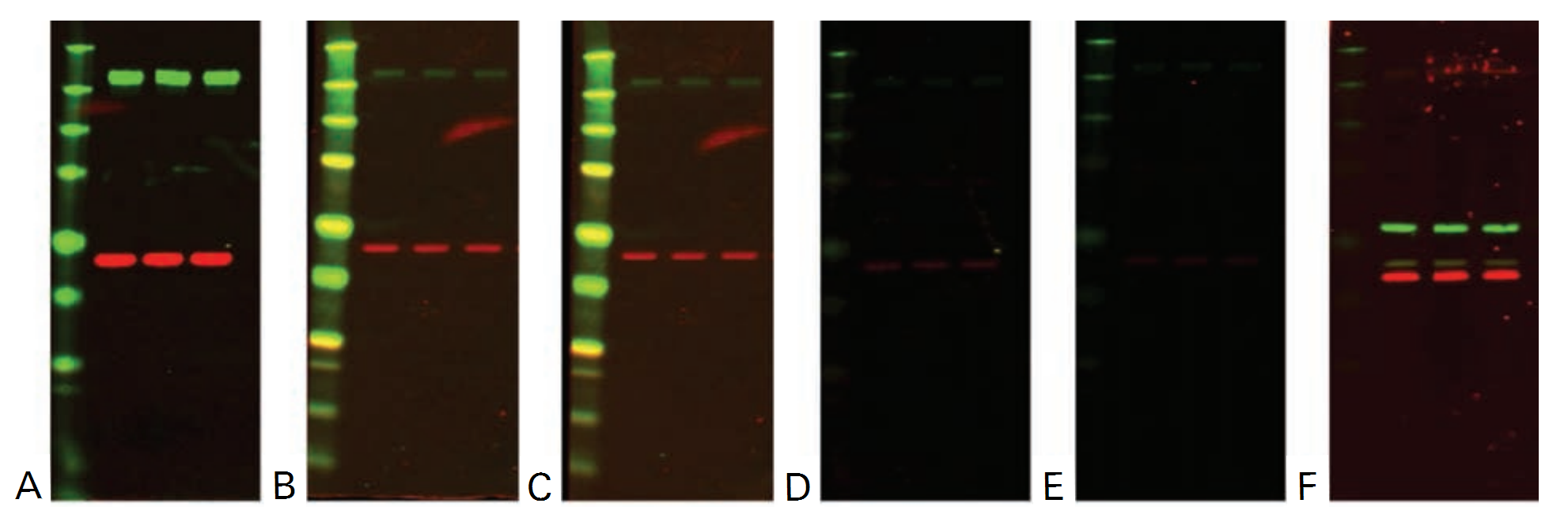
Example Data
The example data shown in the following figures illustrate the relative strip-and-reprobe effectiveness of increasing time and addition of SDS. The sample and protein targets, in this case, were not sufficiently stripped using the standard stripping procedure, so optimization was required.